Macroalgal diversity of Santa Cesarea-Castro (Salento Peninsula, southeastern Italy)
Antonella Bottalico1*, Giuseppina Alongi2 & Cesira Perrone1
1 Dipartimento di Biologia, Università degli Studi di Bari “A. Moro”, Via E. Orabona 4, 70125 Bari, Italy; antonella.bottalico@uniba.it
2 Dipartimento di Scienze Biologiche, Geologiche e Ambientali, Università di Catania, Via A. Longo 19, 95125 Catania, Italy
* Corresponding author.
| |
Abstract
Bottalico, A., Alongi, G. & Perrone, C. 2016. Macroalgal diversity of Santa Cesarea-Castro (Salento Peninsula, southeastern
Italy). Anales Jard. Bot. Madrid 73(2): e042.
The benthic macroalgal flora from the eastern Ionian coastal area of the Peninsula Salentina is scarcely studied. This study gives a contribution to the knowledge on its biodiversity in this area, which also includes marine caves, and the geographical
distribution of some interesting species. A total of 174 macroalgae (119 Rhodophyta, 27 Ochrophyta, and 28 Chlorophyta) were
identified. Six species are first records for the region, one of which, Liagora ceranoides, represents a new record for the Italian flora. The vegetation of most of the wave-exposed rocky substrata, as well as of the two sulphureous caves at Santa Cesarea Terme is characterised by extensive populations of Corallinales. The chorological spectrum of the flora shows a high occurrence of Indo-Pacific and Circumtropical elements, thus resulting more similar to that of floras of the Greek Ionian Sea.
Keywords: Apulia,
biodiversity,
Liagora ceranoides,
macroalgae,
marine caves,
tropicalisation.
|
| |
Resumen
Bottalico, A., Alongi, G. & Perrone, C. 2016. Diversidad de macroalgas de Santa Cesarea-Castro (península de Salento, sudeste
de Italia). Anales Jard. Bot. Madrid 73(2): e042.
La flora bentónica marina de la zona costera jónica de la península Salentina, situada en el extremo sudeste de la región italiana de Apulia, está poco estudiada. Este trabajo contribuye al conocimiento de la biodiversidad de algas de esta zona e incluye también cuevas marinas, así como la distribución geográfica de algunas de sus especies más interesantes. Se han encontrado un total de 174 macroalgas (119 Rhodophyta, 27 Ochrophyta y 28 Chlorophyta). Seis especies han sido identificadas por primera vez en la región, una de las cuales, Liagora ceranoides, representa un nuevo registro para la flora italiana. La vegetación característica de la mayoría de los sustratos rocosos expuestos, así como de las dos cuevas sulfurosas de Santa Cesarea Terme, consiste en extensas poblaciones de Corallinales. El espectro corológico de la flora muestra una alta incidencia de elementos indo-pacíficos y circuntropicales, lo que la asimila a las floras del Mar Jónico griego.
Palabras clave: Apulia,
biodiversidad,
cuevas marinas,
Liagora ceranoides,
macroalgas,
tropicalización.
|
INTRODUCTIONTOP
The evaluation of marine macroalgal diversity of Apulia (southeastern Italy) is particularly interesting due to the vast coastline
of this region (994.6 km, including the Tremiti Islands) and its geographical position, acting as a bridge between the east
and the west of the Mediterranean basin. An inventory of the Apulian macroalgal flora, based on the available literature,
was published more than ten years ago by Cormaci & al. (2001) and reported 616 taxa at specific and infraspecific level (37 species inquirendae included), representing about the 65% of
the entire Italian flora (Furnari & al., 2010). The Apulian flora of the Adriatic side resulted to be richer than the Ionian one (569 vs. 450 species). These dissimilarities,
probably due to different coastline extents (~600 vs. 230 km), also definitely reflect the scarce floristic surveys carried
out on the Ionian shores, with several large areas, such as the eastern Ionian side, still remaining poorly explored (Cormaci & al., 2001). Afterwards, very few floristic papers on the Apulian coasts were published (Bottalico & Delle Foglie, 2003; Cecere & al., 2005). New records were occasionally reported (Bottalico & Delle Foglie, 2002; Bottalico & al., 2006) and three new species were described: Parviphycus felicinii C. Perrone & C.I. Delle Foglie (Perrone & Delle Foglie, 2006), Parviphycus albertanoae A. Bottalico & al. (Bottalico & al., 2014), and Parviphycus bompardii A. Bottalico & al. (Bottalico & al., 2015).
The present paper aims to fill up the previously mentioned gap, giving a contribution to the knowledge of the benthic macroalgal
flora along the eastern Ionian coastline between Santa Cesarea Terme and Castro. Both the towns are included in the “Regional
Natural Park of the Otranto-Santa Maria di Leuca Coast and Tricase Wood” and the coastline strip falls within the area that
will soon receive designation as a marine protected area under the name “Peninsula Salentina” (cf. www.minambiente.it). The earliest data from this area are those by Huvé & al. (1963), who reported 19 species collected at Santa Cesarea Terme, whereas a more consistent number of records can be found in Lazzo & al. (2002) and Bottalico & al. (2011). Phytobenthos of the Castro coastline has never been studied to date.
Several marine karstic caves characterised by sharp physical, chemical and hydrodynamic gradients emphasise the value of this
area. The caves represent one of the most important littoral environments in Apulia, the Italian region with the highest density
of marine caves. Submerged and partially submerged marine caves are protected by the EC and listed in Annex I of the Habitat
Directive 92/43/EEC as a habitat of Community Interest. A few studies have addressed the archeological, paleontological and
faunistic importance of such interesting sites and no attempts to inventory macroalgal diversity, with the exception of Grotta
delle Viole (Tremiti Islands, Adriatic Sea) (Pignatti & al., 1967), were made. In order to contribute to the basic knowledge of the phytobenthos of these poorly-known habitats, the benthic
algal flora of three semi-submerged marine caves of the area was also studied.
Thus the present inventory also represents an initial reference that may be useful to the sustainable management of the future
marine protected area, as well as a solid framework and a starting point to understand the current status and possible future
changes in biodiversity that could hereafter occur on the medium and long term.
MATERIAL AND METHODSTOP
Description of study areaTOP
The “Peninsula Salentina”, described as the “heel” of the Italian “boot”, represents the easternmost area of Italy, stretching between the Gulf of Taranto and the Otranto Channel. The western coastline is entirely bordered by the Ionian Sea, whereas
the eastern one by the Adriatic Sea up to the Capo d’Otranto and the Ionian Sea up to the Capo Santa Maria di Leuca. By convention,
the Strait of Otranto represents the boundary between the Adriatic and the Ionian seas. Santa Cesarea Terme is at 6 km south
of the Capo d’Otranto and is located on a cliff opposite the Otranto Channel; Castro is located approximately at 8 km south
of Santa Cesarea Terme. The investigated coastline is characterised by rocky cliffs of Cretaceous limestone exposed to intense
hydrodinamism and occasionally interspersed with short tracts of sandy bottom. The coastline is dominated by a steep slope
extending from approximately +120 m to approximately −50 m. The continental shelf of the Italian coasts notoriously reaches
its maximum slope in this area, with the 50 m isobath very near the coast. The coastal landscape is also marked by several
sea caves, three of which were investigated in the present study: Grotta Grande and Grotta Solfatara at Santa Cesarea Terme,
Grotta Palombara at Castro. Grotta Grande (or Sulfurea) (40°2’11” N, 18°27’50” E) is one of the four karstic caves of Santa
Cesarea Terme, all characterized by sulphur springs, consisting of a semi-submerged elliptical cavity (length 103 m, maximum
depth 1.5 m) with a gentle sloping rocky bed. Grotta Solfatara (40°2’4” N, 18°27’36” E) has a large entrance about 10 m wide
and 5 m high, and it is characterized by dense and lactescent waters when high flow periods of the sulphur spring occur, during
which the visibility is heavily impacted. Grotta Palombara (“Palummara” or “Picciunara” in reference to the pigeons nesting
in the crevices of its stone walls) (40°0’17” N, 18°25’51” E), is a marine cave located at the intersection of two major faults.
The huge cavity (length 76 m, width 18 m) has high vaults (height 30 m) that slope abruptly to a 10 m deep flat bed; the cave
is characterised by very low light levels due to its narrow entrance.
Sampling procedureTOP
The field work was performed along an approximately 9.5 km long coastline strip. The sampling covered all seasons, from September
2012 to August 2013, and from April 2013 to February 2014; with this schedule, it was possible to collect species with different
life strategies. Samples were collected by SCUBA along 7 transects arrayed perpendicularly to the coastline (5 at Santa Cesarea
Terme and 2 at Castro) (Fig. 1, Table 1) from the midlittoral zone to a maximum depth of 30 m, for a total of 216 samples (55 in spring, 57 in summer, 54 in autumn,
and 50 in winter).
Table 1. Sampling sites along the Santa Cesarea Terme-Castro coastline.
| |
Abbreviation |
Latitude (°N) |
Longitude (°E) |
| Santa Cesarea Terme |
| Contrada Malepasso |
CM |
40°3’8” |
18°28’38” |
| Torre Specchialaguardia |
TS |
40°2’39” |
18°28’31” |
| Miramare |
Mi |
40°2’17” |
18°28’3” |
| Archi |
Ar |
40°2’0” |
18°27’17” |
| Porto Miggiano |
PM |
40°1’45” |
18°26’37” |
| Castro |
| Porto Romanelli |
PR |
40°0’58” |
18°26’1” |
| Palombara |
Pa |
40°0’19” |
18°25’52” |
|
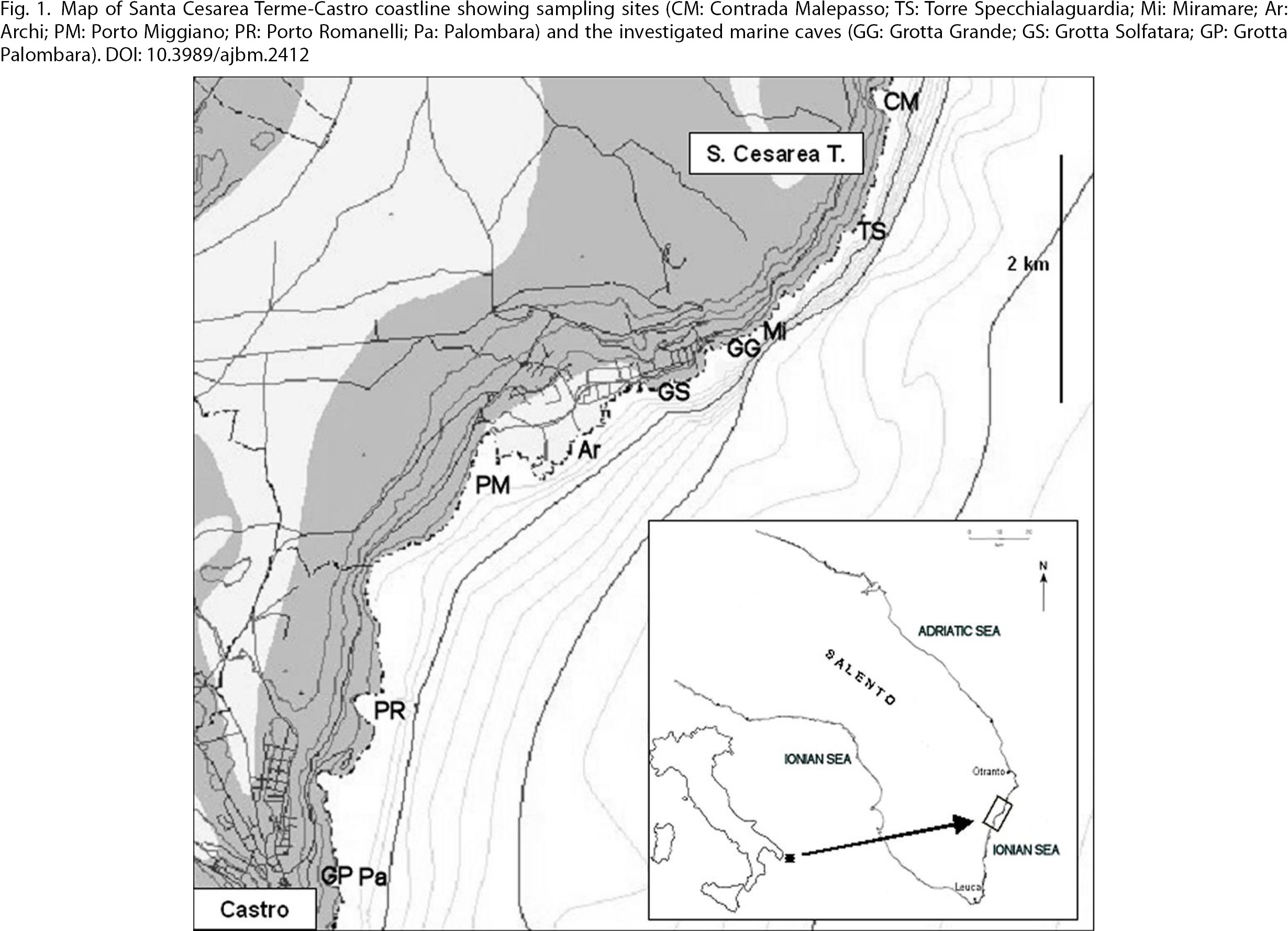 Fig. 1. Map of Santa Cesarea Terme-Castro coastline showing sampling sites (CM: Contrada Malepasso; TS: Torre Specchialaguardia; Mi:
Miramare; Ar: Archi; PM: Porto Miggiano; PR: Porto Romanelli; Pa: Palombara) and the investigated marine caves (GG: Grotta
Grande; GS: Grotta Solfatara; GP: Grotta Palombara). Fig. 1. Map of Santa Cesarea Terme-Castro coastline showing sampling sites (CM: Contrada Malepasso; TS: Torre Specchialaguardia; Mi:
Miramare; Ar: Archi; PM: Porto Miggiano; PR: Porto Romanelli; Pa: Palombara) and the investigated marine caves (GG: Grotta
Grande; GS: Grotta Solfatara; GP: Grotta Palombara).
|
|
The sampling inside the caves (Fig. 1) was carried out in the same periods and samples were collected randomly from the entrance up to approximately 10 m inwards
each cave. Sample collection both into the caves and on open coast was made by hand or with a chisel in order to remove basal
portions, encrusting algae or mat-forming algae, attempting to gather as many species as possible by sampling different microhabitats.
Fresh material was observed under a Leica MZ 7.5 steromicroscope (Leica, Wetzlar, Germany). Sections of thalli were obtained
by hand or, if necessary, with a DSK-1000 vibratome (Dosaka, Kyoto, Japan) and properly stained. Photomicrographs and measurements
were made using an Olympus BX-40 light microscope (Olympus, Melville, USA) fitted with a Nikon Coolpix 990 digital camera
(Nikon, Tokyo, Japan). The collected material was preserved in 4% buffered formalin/seawater and/or prepared as herbarium
specimens. The most interesting exsiccata were deposited in the Herbarium Horti Botanici Barensis (BI, Italy). Herbarium abbreviation
follows Thiers (2014). For nomenclature purposes, the following taxonomic databases were used: Index Nominum Algarum (Silva, 2014) and AlgaeBase (Guiry & Guiry, 2014). After sorting, macroalgal taxa were identified according to the methodology of Cormaci & al. (2004). In the floristic list (Table 2) for each species sampling stations, bathimetric distribution and phytogeographic elements (according to Furnari & al., 2010) were given.
Table 2. List of macroalgal flora from the Santa Cesarea Terme-Castro coastline and caves. Species reported for the first time either in Italy or in Apulia or noteworthy as second records for the Apulian coasts (see Discussion) are marked with I, A
and Sr, respectively. In the Chorology column, phytogeographic elements are named according to Furnari & al. (2010): A: Atlantic;
Ab: Boreo-Atlantic; Abt: Boreo-tropical Atlantic; AP: Atlanto-Pacific; APt: Atlanto-Pacific tropical; APct: Atlanto-Pacific
cold temperate; At: Atlantic tropical; C: Cosmopolite; CB: Circumboreal; CT: Circumtropical; IA: Indo-Atlantic; IAct: Indo-Atlantic
cold temperate; IAt: Indo-Atlantic tropical; IP: Indo-Pacific; M: Mediterranean; SC: Sub-cosmopolitan. The occurrence of every
species in the 7 coastline sites (CM, TS, Mi, Ar, PM, PR, Pa) and the 3 caves (GG, GS, GP) is marked *. For the bathymetric distribution, the following abbreviations are used: M = Midlittoral zone; I = Infralittoral zone; uI = upper Infralittoral zone; lI = lower Infralittoral zone.
| Chorology |
Taxa |
Santa Cesarea Terme |
Castro |
Bathymetric distribution |
| CM |
TS |
Mi |
Ar |
PM |
GG |
GS |
PR |
Pa |
GP |
| |
RHODOPHYTA |
|
|
|
|
|
|
|
|
|
|
|
| IA |
SrAcrochaetium microscopicum (Nägeli ex Kütz.) Nägeli |
* |
|
|
* |
|
|
|
|
|
|
M |
| M |
Acrochaetium minutissimum (Shur) Nägeli |
|
|
|
* |
* |
|
|
* |
|
|
M |
| IA |
Acrosorium ciliolatum (Harv.) Kylin |
|
|
|
* |
|
|
|
|
|
|
I |
| M |
SrAcrosymphyton purpuriferum (J. Agardh) G. Sjöstedt |
|
|
|
* |
|
|
|
|
|
|
I |
| IP |
AAcrothamnion preissii (Sond.) E.M. Woll. |
|
|
|
|
* |
|
|
|
* |
|
I |
| At |
Alsidium corallinum C. Agardh |
|
* |
|
|
* |
|
|
|
|
|
M, I |
| CT |
Amphiroa cryptarthrodia Zanardini |
|
* |
* |
* |
|
* |
* |
|
|
|
I |
| SC |
Amphiroa rigida J.V. Lamour. |
|
|
|
|
|
* |
|
* |
|
|
I |
| IA |
Antithamnion cruciatum (C. Agardh) Nägeli |
|
* |
* |
* |
|
|
|
|
|
|
M, I |
| IA |
Apoglossum ruscifolium (Turner) J. Agardh |
* |
|
|
* |
|
|
* |
|
|
|
I |
| SC |
AAsparagopsis taxiformis (Delile) Trevis. (only as Falkenbergia phase) |
|
* |
* |
* |
* |
|
|
|
|
|
M, I |
| Ab |
Boergeseniella fruticulosa (Wulfen) Kylin |
|
|
|
* |
* |
|
|
|
|
|
uI |
| Ab |
SrBornetia secundiflora (J. Agardh) Thur. |
* |
|
|
|
|
|
|
* |
|
|
uI |
| IA |
Botryocladia botryoides (Wulfen) Feldmann |
* |
* |
* |
|
|
|
|
* |
|
|
lI |
| IA |
Callithamnion granulatum (Ducluz.) C. Agardh |
|
|
|
* |
|
|
|
* |
|
|
M, uI |
| M |
Ceramium ciliatum var. robustum (J. Agardh) Feldm.-Maz. |
|
* |
* |
* |
|
|
|
|
|
|
M, uI |
| SC |
Ceramium diaphanum (Lightf.) Roth |
|
|
|
* |
|
|
|
* |
|
|
M, I |
| M |
SrCeramium giacconei Cormaci & G. Furnari |
|
|
|
* |
|
|
|
|
|
|
I |
| M |
Ceramium incospicuum Zanardini |
|
|
|
* |
* |
|
|
|
|
|
M, uI |
| SC |
Ceramium siliquosum (Kütz.) Maggs & Hommers |
* |
* |
|
* |
|
|
|
|
|
|
M, I |
| SC |
Ceramium tenerrimum (G. Martens) Okamura |
|
|
|
* |
* |
|
|
|
|
|
M, I |
| SC |
Ceramium virgatum Roth |
|
|
* |
* |
|
|
|
|
|
|
M |
| C |
Champia parvula (C. Agardh) Harv. |
* |
|
|
* |
|
|
|
|
|
|
I |
| C |
Chondracanthus acicularis (Roth) Fredericq |
|
|
|
* |
* |
|
|
|
|
|
uI |
| Ab |
Chondria coerulescens (J. Agardh) Falkenb. |
|
* |
* |
* |
* |
|
|
|
|
|
M |
| At |
Chrysymenia ventricosa (J.V. Lamour.) J. Agardh |
* |
|
|
|
|
|
|
|
* |
|
I |
| SC |
Colaconema savianum (Menegh.) R. Nielsen |
* |
|
|
* |
|
|
|
|
|
|
M |
| SC |
Corallina officinalis L. |
* |
* |
|
|
|
* |
|
|
|
|
M, uI |
| SC |
Crouania attenuata (C. Agardh) J. Agardh |
|
|
* |
|
|
|
|
* |
* |
|
M, I |
| IA |
Cryptonemia lomation (Bertol.) J. Agardh |
* |
|
|
* |
|
|
* |
* |
|
|
lI |
| Abt |
Dasya rigidula (Kütz.) Ardiss. |
|
|
|
* |
|
|
|
|
* |
|
M, I |
| A |
Ellisolandia elongata (J. Ellis & Sol.) K.R. Hind & G.W. Saunders |
|
|
|
* |
|
* |
* |
|
|
|
M, uI |
| SC |
Erythrocladia irregularis Rosenv. |
* |
|
|
|
|
|
|
|
|
|
uI |
| C |
Erythrotrichia carnea (Dillwyn) J. Agardh |
|
* |
|
* |
* |
|
|
|
|
|
M, I |
| IA |
Eupogodon planus (C. Agardh) Kütz. |
* |
|
|
|
|
|
|
|
* |
|
lI |
| Ab |
Gastroclonium clavatum (Roth) Ardiss. |
|
* |
* |
|
|
|
|
* |
* |
|
M, uI |
| C |
Gayliella mazoyerae T.O. Cho & al. |
|
|
|
* |
* |
|
|
|
|
|
M, I |
| IP |
Gelidiella lubrica (Kütz.) Feldmann & Hamel |
|
|
|
* |
|
|
|
|
|
|
M, uI |
| |
Gelidium sp. |
|
|
|
* |
|
|
|
|
|
|
uI |
| Ab |
Gelidium bipectinatum G. Furnari |
|
|
|
* |
|
|
|
|
|
|
M, uI |
| SC |
Gelidium crinale (Hare ex Turner) Gaillon |
* |
|
|
* |
|
|
|
|
|
|
M, uI |
| Ab |
Gelidium spathulatum (Kütz.) Bornet |
|
|
|
* |
|
|
|
|
|
|
M |
| SC |
Gelidium spinosum (S.G. Gmel.) P.C. Silva |
* |
|
|
* |
|
|
|
|
|
|
M, I |
| IA |
Griffithsia opuntioides J. Agardh |
|
|
|
|
|
|
|
* |
|
|
uI |
| Abt |
Griffithsia phyllamphora J. Agardh |
|
|
|
|
|
|
|
* |
|
|
uI |
| IA |
Halopithys incurva (Huds.) Batters |
|
* |
* |
* |
|
|
|
* |
|
|
lI |
| SC |
Halymenia floresii (Clemente) C. Agardh |
|
|
|
|
|
|
|
* |
|
|
I |
| Abt |
Haraldia lenormandii (Derbès & Solier) Feldmann |
|
|
|
|
|
|
|
* |
|
|
uI |
| CT |
Herposiphonia secunda (C. Agardh) Ambronn |
|
|
|
* |
* |
|
|
|
|
|
M, I |
| SC |
Hildenbrandia rubra (Sommerf.) Menegh. |
* |
* |
|
* |
|
|
* |
|
|
* |
M, uI |
| C |
Hydrolithon boreale (Foslie) Y.M. Chamb. |
* |
|
|
|
|
* |
* |
|
|
* |
M, I |
| C |
Hydrolithon farinosum (J.V. Lamour.) Penrose & Y.M. Chamb. |
|
* |
* |
* |
|
* |
|
|
|
|
M, I |
| CT |
Hypnea musciformis (Wulfen) J.V. Lamour. |
* |
|
|
* |
|
|
|
|
|
|
M, uI |
| CT |
Hypnea spinella (C. Agardh) Kütz. |
* |
|
|
* |
* |
|
|
|
|
|
M, uI |
| Ab |
Hypoglossum hypoglossoides (Stackh.) Collins & Herv. |
* |
|
|
* |
|
|
|
|
* |
|
I |
| IA |
Jania longifurca Zanardini |
|
|
|
* |
|
* |
* |
|
|
|
uI |
| C |
Jania rubens (L.) J.V. Lamour. var. rubens |
|
|
|
* |
|
|
|
|
|
|
M, uI |
| Ab |
Jania rubens var. corniculata (L.) Yendo |
* |
* |
* |
* |
|
|
|
|
|
|
uI |
| IAct |
Jania virgata (Zanardini) Mont. |
* |
* |
* |
|
|
* |
* |
|
|
|
I |
| SC |
Kallymenia reniformis (Turner) J. Agardh |
|
|
|
* |
|
|
|
* |
|
|
lI |
| |
Laurencia sp. |
|
|
|
* |
* |
|
|
|
|
|
M, uI |
| IP |
Laurencia glandulifera (Kütz.) Kütz. |
|
|
|
* |
* |
|
|
|
|
|
M, uI |
| IA |
Laurencia intricata J.V. Lamour. |
|
|
|
* |
* |
|
|
|
|
|
M, uI |
| IP |
Laurencia microcladia Kütz. |
|
* |
* |
|
* |
|
|
* |
|
|
uI |
| C |
Laurencia obtusa (Huds.) J.V. Lamour. |
|
|
|
* |
* |
|
|
|
|
|
M, uI |
| SC |
ILiagora ceranoides J.V. Lamour. |
|
|
|
* |
|
|
|
|
|
|
uI |
| SC |
ALiagora distenta (Mert. ex Roth) J.V. Lamour. |
|
|
|
* |
|
|
|
|
|
|
uI |
| SC |
Liagora viscida (Forssk.) C. Agardh |
|
|
|
* |
|
|
|
|
|
|
uI |
| IP |
Lithophyllum byssoides (Lam.) Foslie |
* |
|
|
* |
|
|
|
|
|
* |
M |
| Ab |
Lithophyllum cystoseirae (Hauck) Heydr. |
|
|
|
* |
|
|
|
|
|
|
M |
| Ab |
Lithophyllum incrustans Phil. |
|
* |
* |
* |
|
* |
* |
|
|
* |
M |
| Abt |
Lithophyllum papillosum (Zanardini ex Hauck) Foslie |
|
|
|
* |
|
* |
|
|
|
|
M |
| IA |
Lithophyllum stictaeforme (Aresch.) Hauck |
|
|
|
* |
* |
* |
|
|
|
|
M, uI |
| IA |
Lophosiphonia reptabunda (Suhr) Kylin |
|
* |
* |
|
* |
|
|
|
|
|
M, I |
| SC |
Melobesia membranacea (Esper) J.V. Lamour. |
* |
|
|
|
* |
* |
* |
|
|
|
M |
| SC |
Nemalion helminthoides (Velley) Batters |
|
|
|
* |
* |
|
|
|
* |
|
M |
| M |
Nemastoma dichotomum J. Agardh |
|
|
|
* |
|
|
|
|
|
|
M, uI |
| SC |
Neogoniolithon brassica-florida (Harv.) Setch. & L.R. Mason |
|
* |
* |
* |
|
* |
* |
|
|
|
M, uI |
| IA |
Nitophyllum punctatum (Stackh.) Grev. |
|
|
|
* |
|
|
|
|
* |
|
uI |
| Ab |
Osmundea truncata (Kütz.) K.W. Nam & Maggs |
* |
|
|
* |
|
|
|
|
|
|
M, uI |
| M |
Osmundea verlaquei G. Furnari |
|
|
|
* |
|
|
|
|
|
|
M, uI |
| C |
Palisada perforata (Bory) K.W. Nam |
* |
|
|
* |
|
|
|
|
|
|
M, uI |
| SC |
Palisada thuyoides (Kütz.) V. Cassano & al. |
|
|
|
* |
|
|
|
|
* |
|
M, uI |
| |
Parviphycus sp. |
|
|
|
* |
* |
|
|
* |
|
|
M, uI |
| IP |
Parviphycus antipae (Celan) Santel. |
|
|
|
* |
|
|
|
|
|
|
uI |
| M |
Peyssonnelia bornetii Boudour. & Denizot |
* |
|
|
* |
|
|
|
* |
|
* |
lI |
| SC |
Peyssonnelia polymorpha (Zanardini) F. Schmitz |
|
|
|
* |
|
* |
|
|
|
* |
I |
| M |
Peyssonnelia rosa-marina Boudour. & Denizot |
|
|
|
* |
* |
|
|
|
|
* |
I |
| IA |
Peyssonnelia rubra (Grev.) J. Agardh |
* |
|
|
* |
* |
|
|
|
|
* |
I |
| M |
Peyssonnelia squamaria (S.G. Gmel.) Decne. |
|
* |
* |
* |
|
* |
* |
* |
|
|
I |
| Ab |
Phymatolithon calcareum (Pall.) W.H. Adey & D.L. McKibbin |
* |
|
|
|
|
* |
* |
|
|
* |
M, uI |
| CB |
Phymatolithon lenormandii (Aresch.) W.H. Adey |
* |
|
|
|
|
* |
|
|
|
* |
M, I |
| Ab |
Platoma cyclocolpum (Mont.) F. Schmitz |
* |
|
|
|
|
|
|
* |
|
|
M, uI |
| SC |
Plocamium cartilagineum (L.) P.S. Dixon |
|
* |
* |
* |
|
|
|
|
* |
|
uI |
| C |
Pneophyllum fragile Kütz. |
|
|
|
* |
|
* |
* |
|
|
|
M, I |
| APct |
Polysiphonia furcellata (C. Agardh) Harv. |
|
|
|
* |
* |
|
|
|
|
|
uI |
| Ab |
Polysiphonia opaca (C. Agardh) Moris & De Not. |
* |
|
|
* |
|
|
|
|
|
|
M, uI |
| CT |
Polysiphonia scopulorum Harv. |
|
|
|
* |
|
|
|
|
|
|
M, uI |
| Ab |
Polysiphonia subulifera (C. Agardh) Harv. |
|
* |
* |
* |
* |
|
|
|
|
|
uI |
| M |
Polysiphonia tripinnata J. Agardh |
* |
|
|
* |
|
|
|
|
|
|
lI |
| SC |
Pterocladiella capillacea (S.G. Gmel.) Santel. & Hommers. |
|
|
|
* |
|
|
|
|
* |
|
M, uI |
| Abt |
Pterocladiella melanoidea (Schousb. ex Bornet) Santelices & Hommers. |
|
|
|
* |
|
|
|
|
|
|
M |
| SC |
Pterosiphonia pennata (C. Agardh) Sauv. |
|
* |
* |
|
|
|
|
* |
|
|
uI |
| M |
SrRadicilingua adriatica (Kylin) Papenfuss |
* |
|
|
* |
|
|
|
|
|
|
I |
| Ab |
Radicilingua reptans (Kylin) Papenfuss |
* |
* |
|
|
|
|
|
|
* |
|
I |
| Ab |
Rhodophyllis divaricata (Stackhouse) Papenf. |
|
* |
* |
* |
* |
|
|
|
|
|
uI |
| Ab |
Rhodymenia ardissonei (Kuntze) Feldmann |
* |
|
|
* |
|
|
|
|
|
|
M, uI |
| IAt |
Rytiphlaea tinctoria (Clemente) C. Agardh |
* |
|
|
* |
* |
|
|
|
|
|
lI |
| IA |
Schottera nicaeensis (J.V. Lamour. ex Duby) Guiry & Hollenb. |
|
|
|
* |
|
|
|
|
|
* |
M, uI |
| AP |
Scinaia furcellata (Turner) J. Agardh |
|
* |
* |
|
* |
|
|
|
|
|
uI |
| M |
Sebdenia rodrigueziana (Feldmann) Athanas. |
|
|
|
|
|
|
|
* |
|
|
lI |
| M |
Seirospora giraudyi (Kütz.) De Toni |
* |
|
|
* |
|
|
|
|
|
|
I |
| Ab |
Sphaerococcus coronopifolius Stackh. |
* |
|
|
* |
|
|
|
|
|
|
I |
| IP |
Spongites fruticulosus Kütz. |
|
|
|
* |
|
|
* |
* |
|
|
M, uI |
| C |
Stylonema alsidii (Zanardini) K.M. Drew |
|
* |
* |
|
|
|
|
* |
|
|
M, I |
| IP |
Taenioma nanum (Kütz.) Papenf. |
|
|
|
* |
|
|
|
|
|
|
uI |
| Abt |
Titanoderma trochanter (Bory) Benhissoune & al. |
|
|
|
* |
|
|
|
|
|
|
M |
| CT |
Tricleocarpa fragilis (L.) Huisman & R.A. Towns. |
|
|
|
* |
|
|
|
|
|
|
uI |
| CT |
Wrangelia penicillata (C. Agardh) C. Agardh |
|
|
|
* |
* |
|
|
|
|
|
uI |
| OCHROPHYTA |
|
|
|
|
|
|
|
|
|
|
|
|
| Ab |
Arthrocladia villosa (Huds.) Duby |
* |
* |
* |
|
|
|
|
|
|
|
uI |
| IA |
Cladostephus spongiosus (Huds.) C. Agardh |
|
* |
* |
|
|
|
|
|
|
|
uI |
| C |
Colpomenia sinuosa (Mert. ex Roth) Derbès & Solier |
|
|
|
* |
* |
|
|
|
|
|
uI |
| CB |
Cutleria adspersa (Mert. ex Roth) De Not. |
|
|
|
* |
|
|
|
* |
|
|
I |
| SC |
Cutleria multifida (Turner) Grev. |
* |
* |
* |
* |
|
|
|
|
* |
|
I |
| M |
Cystoseira amentacea var. stricta Mont. |
|
|
|
* |
* |
|
|
|
|
|
M, uI |
| Ab |
Cystoseira compressa (Esper) Gerloff & Nizam. |
|
|
|
* |
|
|
|
|
|
|
uI |
| C |
Dictyopteris polypodioides (DC.) J.V. Lamour. |
* |
* |
* |
* |
* |
|
|
|
* |
|
uI |
| C |
Dictyota dichotoma (Huds.) J.V. Lamour. |
* |
|
|
* |
|
|
|
* |
* |
|
I |
| SC |
Dictyota dichotoma var. intricata (C. Agardh) Grev. |
* |
|
|
* |
|
|
|
|
|
|
I |
| SC |
Dictyota fasciola (Roth) J.V. Lamour. |
|
|
|
* |
|
|
|
|
|
|
I |
| SC |
Dictyota linearis (C. Agardh) Grev.1
|
|
|
|
* |
|
|
|
|
|
|
I |
| A |
Dictyota spiralis Mont. |
|
* |
* |
* |
|
|
|
|
|
|
I |
| APct |
SrElachista flaccida (Dillwyn) Fr. |
* |
|
|
|
* |
|
|
|
|
|
uI |
| SC |
Halopteris filicina (Gratel.) Kütz. |
|
|
|
* |
* |
|
|
|
|
|
I |
| SC |
Halopteris scoparia (L.) Sauv. |
* |
* |
* |
* |
|
|
|
|
* |
|
I |
| Ab |
Myriactula stellulata (Harv.) Levring |
* |
|
|
* |
|
|
|
|
|
|
lI |
| CT |
APadina ditristromatica Ni-Ni-Win & H. Kawai |
|
* |
* |
* |
|
|
|
|
|
|
I |
| CT |
Padina pavonica (L.) Thivy |
* |
* |
* |
* |
* |
|
|
* |
* |
|
I |
| SC |
Ralfsia verrucosa (Aresch.) Aresch. |
|
|
|
* |
* |
|
|
|
|
|
M |
| C |
Scytosiphon lomentaria (Lyngb.) Link |
|
|
|
* |
|
|
|
|
|
|
M |
| SC |
Sphacelaria cirrosa (Roth) C. Agardh |
* |
|
|
* |
|
|
|
|
|
|
M, I |
| SC |
Sphacelaria fusca (Huds.) Gray |
|
|
|
* |
|
|
|
|
|
|
I |
| Ab |
Sphacelaria plumula Zanardini |
|
* |
* |
* |
|
|
|
|
|
|
I |
| M |
Stictyosiphon adriaticus Kütz. |
* |
* |
* |
|
|
|
|
* |
|
|
I |
| IA |
Taonia atomaria (Woodw.) J. Agardh |
|
* |
* |
|
* |
|
|
|
|
|
I |
| AP |
Zanardinia typus (Nardo) P.C. Silva |
* |
* |
|
* |
|
|
|
|
* |
|
I |
| CHLOROPHYTA |
|
|
|
|
|
|
|
|
|
|
|
|
| IA |
Acetabularia acetabulum (L.) P.C. Silva |
|
* |
* |
|
|
|
|
|
* |
|
M, uI |
| CT |
Anadyomene stellata (Wulfen) C. Agardh |
|
|
|
* |
|
|
|
* |
|
|
uI |
| AP |
Bryopsis corymbosa J. Agardh |
|
* |
* |
* |
|
|
|
|
|
|
M, uI |
| M |
Bryopsis cupressina J.V. Lamour. |
* |
|
|
* |
|
|
|
|
|
|
M, uI |
| A |
Bryopsis duplex De Not. |
|
|
|
* |
|
|
|
|
* |
|
M |
| SC |
Bryopsis plumosa (Huds.) C. Agardh |
|
* |
* |
* |
|
|
|
|
|
|
uI |
| IA |
Cladophora coelothrix Kütz. |
* |
|
|
|
|
|
|
|
|
|
uI |
| IA |
Cladophora dalmatica Kütz. |
* |
* |
|
* |
|
|
|
|
|
|
M, uI |
| IA |
Cladophora echinus (Biasol.) Kütz. |
|
|
|
* |
* |
|
|
|
|
|
uI |
| IA |
Cladophora flexuosa (O.F. Müll.) Kütz. |
|
|
|
|
* |
|
|
|
* |
|
M, uI |
| IA |
Cladophora pellucida (Huds.) Kütz. |
|
* |
* |
* |
|
|
|
|
|
|
M, uI |
| IA |
Cladophora prolifera (Roth) Kütz. |
|
|
|
* |
|
|
|
|
|
|
I |
| AP |
Cladophora rupestris (L.) Kütz. |
|
|
|
* |
|
|
|
|
* |
|
M, uI |
| Abt |
Codium bursa (Olivi) C. Agardh |
* |
* |
|
* |
|
|
|
* |
* |
|
I |
| IP |
Codium effusum (Raf.) Delle Chiaje |
* |
|
* |
|
|
|
* |
* |
|
* |
I |
| At |
Codium vermilara (Olivi) Delle Chiaje |
|
|
|
* |
|
|
|
* |
|
|
I |
| At |
Dasycladus vermicularis (Scop.) Krasser |
|
|
|
* |
|
|
|
|
|
|
M |
| SC |
Derbesia tenuissima (Moris & De Not.) P. Crouan & H. Crouan (also as gametophyte Halicystis parvula) |
|
* |
|
* |
|
|
|
|
* |
|
M, I |
| At |
Flabellia petiolata (Turra) Nizam. |
* |
* |
* |
* |
|
|
|
* |
|
* |
I |
| CT |
Halimeda tuna (J. Ellis & Sol.) J.V. Lamour. |
* |
|
|
* |
|
|
* |
|
|
* |
I |
| APt |
Palmophyllum crassum (Naccari) Rabenh. |
* |
|
|
|
|
|
|
* |
|
* |
lI |
| M |
APseudobryopsis myura (J. Agardh) Berthold & Oltm. |
* |
|
|
* |
|
|
|
|
|
|
lI |
| C |
Ulva laetevirens Aresch. |
|
|
|
* |
|
|
|
|
|
|
M, uI |
| C |
Ulva linza L. |
|
* |
* |
* |
|
|
|
|
|
|
M, uI |
| SC |
Ulva prolifera O.F. Müll. |
|
* |
|
* |
|
|
|
|
|
|
M, uI |
| Ab |
Ulva rotundata Bliding |
|
* |
* |
* |
|
|
|
|
|
|
M, uI |
| C |
Ulvella viridis (Reinke) R. Nielsen & al. |
|
|
|
* |
|
|
|
|
|
|
M, I |
| CT |
Valonia utricularis (Roth) C. Agardh |
|
|
|
* |
|
|
|
|
* |
|
M, I |
| 1 We preferred to maintain the nomenclature followed by Cormaci & al. (2012) until the synonymy with D. implexa (Desf.) J.V. Lamour. (Tronholm & al., 2010) is resolved. |
RESULTSTOP
We identified 174 taxa (later referred to as “species” for convenience) comprising 119 Rhodophyta (68.4%), 27 Ochrophyta (15.5%),
and 28 Chlorophyta (16.1%) (Table 2). With regards to the macroalgae collected in the caves, 31 species (27 Rhodophyta and 4 Chlorophyta) were recorded, all
falling within the whole floristic contingent of the investigated coastline (Table 2). In particular, the flora of Grotta Grande comprised 18 species, all belonging to Rhodophyta; in the Grotta Solfatara were
recorded 17 species (15 Rhodophyta and 2 Chlorophyta); the flora of Grotta Palombara comprised 15 species (11 Rhodophyta and
4 Chlorophyta).
The three taxa identified at generic level were also interesting and will be put through further deeper investigations: the
first is a species of Laurencia J.V. Lamour. erroneously reported in past collections as Laurencia majuscula (Harvey) A.H.S. Lucas (Lazzo & al., 2002; Bottalico & al., 2011); the second is a diminuitive species belonging to a species of Gelidium J.V. Lamour. probably new to the Mediterranean region (unpublished data); the third one belongs to Parviphycus Santel.
From a biogeographic point of view, the flora of Santa Cesarea Terme-Castro was characterised by a high incidence of Atlantic
elements (42.94%), followed by Cosmopolitan (31.76%) and Mediterranean elements (11.18%). A lower incidence was shown by the
Circumtropical (7.06%), the Indo-Pacific (5.88%) and the Circumboreal (1.18%) elements (Table 2). This spectrum is different from that one recently calculated by Furnari & al. (2010) for the Italian flora, mainly for
the Mediterranean (11.18 vs. 25.74%) and Cosmopolitan elements (31.76 vs. 21.51%). On the contrary, the Indo-Pacific and Circumtropical
elements globally considered, have a percentage value (12.94%) much higher than that reported for both the Apulian floristic
contingent (8.8%) (Cormaci & al., 2001) and the entire Italian flora (9.09%) (Furnari & al., 2010), but very similar to that of floras of the eastern Ionian Sea (12.50%). Of the 33 species alien to the Mediterranean Sea
and occurring along the Italian coast (Occhipinti-Ambrogi & al., 2011), only two were found at Santa Cesarea Terme-Castro: Acrothamnion preissii (Sond.) E.M. Woll. and Hypnea spinella (C. Agardh) Kütz. No alien species occurred in the caves.
Of the 174 taxa, the following records are noteworthy and interesting from a phytogeographic point of view:
Acrothamnion preissii (Sond.) E.M. Woll.
Vegetative thalli (Fig. 2a), showing characteristic apical gland cells (Fig. 2b), and tetrasporic thalli (Fig. 2c) were collected at both Santa Cesarea Terme and Castro. Our specimens are in good agreement with both Australian (Womersley, 1998) and Italian specimens from Leghorn (Cinelli & Sartoni, 1969). This species is considered as particularly invasive in the central Tyrrhenian Sea (Klein & Verlaque, 2011) and has already colonised many parts of the western Mediterranean basin (Boillot & al., 1982; Ferrer & al., 1994; Piazzi & al., 1996) after its arrival near Leghorn (Tuscany, Italy) (Cinelli & Sartoni, 1969). More recently it was reported from the Croatian coast (Žuljevic & al., 2009) and Sicily (Alongi & al., 2012). The Apulian record extends its distribution area to the eastern Ionian Sea.
|
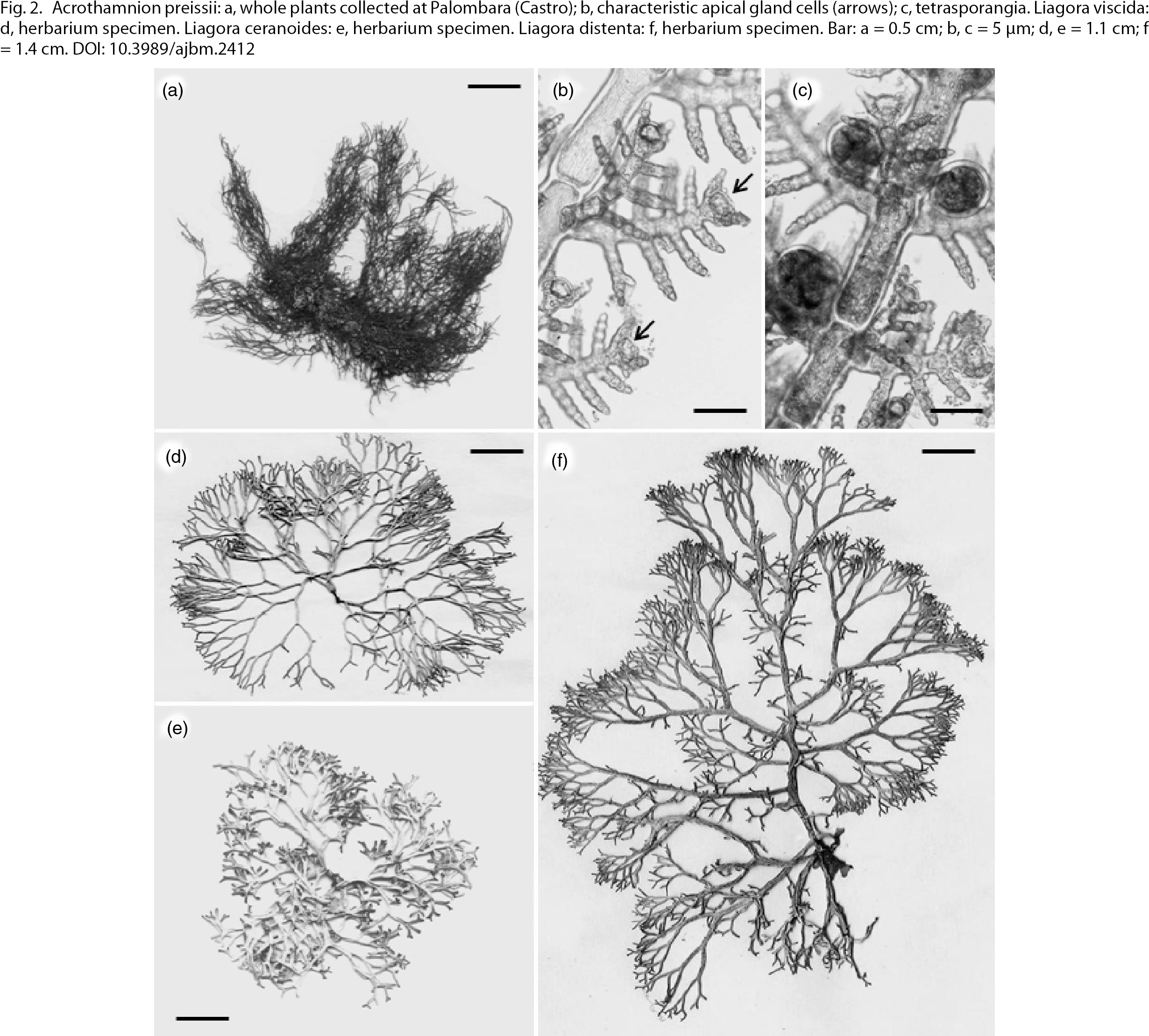 Fig. 2. Acrothamnion preissii: a, whole plants collected at Palombara (Castro); b, characteristic apical gland cells (arrows); c, tetrasporangia. Liagora viscida: d, herbarium specimen. Liagora ceranoides: e, herbarium specimen. Liagora distenta: f, herbarium specimen. Bar: a = 0.5 cm; b, c = 5 μm; d, e = 1.1 cm; f = 1.4 cm. Fig. 2. Acrothamnion preissii: a, whole plants collected at Palombara (Castro); b, characteristic apical gland cells (arrows); c, tetrasporangia. Liagora viscida: d, herbarium specimen. Liagora ceranoides: e, herbarium specimen. Liagora distenta: f, herbarium specimen. Bar: a = 0.5 cm; b, c = 5 μm; d, e = 1.1 cm; f = 1.4 cm.
|
|
Asparagopsis taxiformis (Delile) Trevis.
Falkenbergia tetrasporophyte stages of Asparagopsis spp. with the classical pompom-like morphology were found in almost all the sampling sites of Santa Cesarea Terme (with the
exception of Contrada Malepasso), either free floating or as epiphyte. Two species, A. armata Harvey and A. taxiformis, are currently reported from the Mediterranean Sea, but to date gametophytes have never been collected in Apulia, where only
the Falkenbergia phase, arbitrarily attributed to A. armata occurs (Cormaci & al., 2001). In the past, tetrasporophyte identification was based only on inference from the presence of the respective gametophytes
or by DNA sequencing (Andreakis & al., 2004; Ní Chualaín & al., 2004), but in a recent paper, Zanolla & al. (2014) proposed a set of useful diagnostic characters to discriminate them. A combination of tetrasporophyte morphological characters
observed in our specimens allowed us to assign them to A. taxiformis which is a new record for Apulia and features an invasive behaviour at Santa Cesarea Terme.
Liagora distenta (Mert. ex Roth) J.V. Lamour.
Three species of the genus Liagora, L. viscida (Forssk.) C. Agardh (Fig. 2d), L. ceranoides (Fig. 2e), and L. distenta (Fig. 2f), were collected in the late spring and summer at Archi (Santa Cesarea Terme). They were easily distinguished based on vegetative
and reproductive characters (Kvaternik & Afonso-Carrillo, 1995; Lin & al., 2013). Male and female thalli of L. distenta were collected in sheltered places from the surface down to −10 m. Thalli, up to 20 cm high, were greenish white, with pink
to red extremities and numerous adventitious branches. The morphology of the assimilatory filaments, with cells becoming shorter
and broader upwards, and ending in pyriform cells (Fig. 3a), was a consistent feature in all the examined plants. Spermatangia occurred on small spermatangial mother cells formed on
the terminal cells of the assimilatory filaments (Fig. 3b). The carpogonial branch is borne on the inner part of the assimilatory filaments (Fig. 3c). This species was previously recorded from Campania, Sardinia, Sicily (Furnari & al., 2003) and Tuscany (Rindi & al., 2002); the present record extends its distribution area to the eastern Italian coast. It is well known, however, that Ardissone (1883) had already reported both L. ceranoides and L. distenta from the Dalmatian coast of the Adriatic Sea.
|
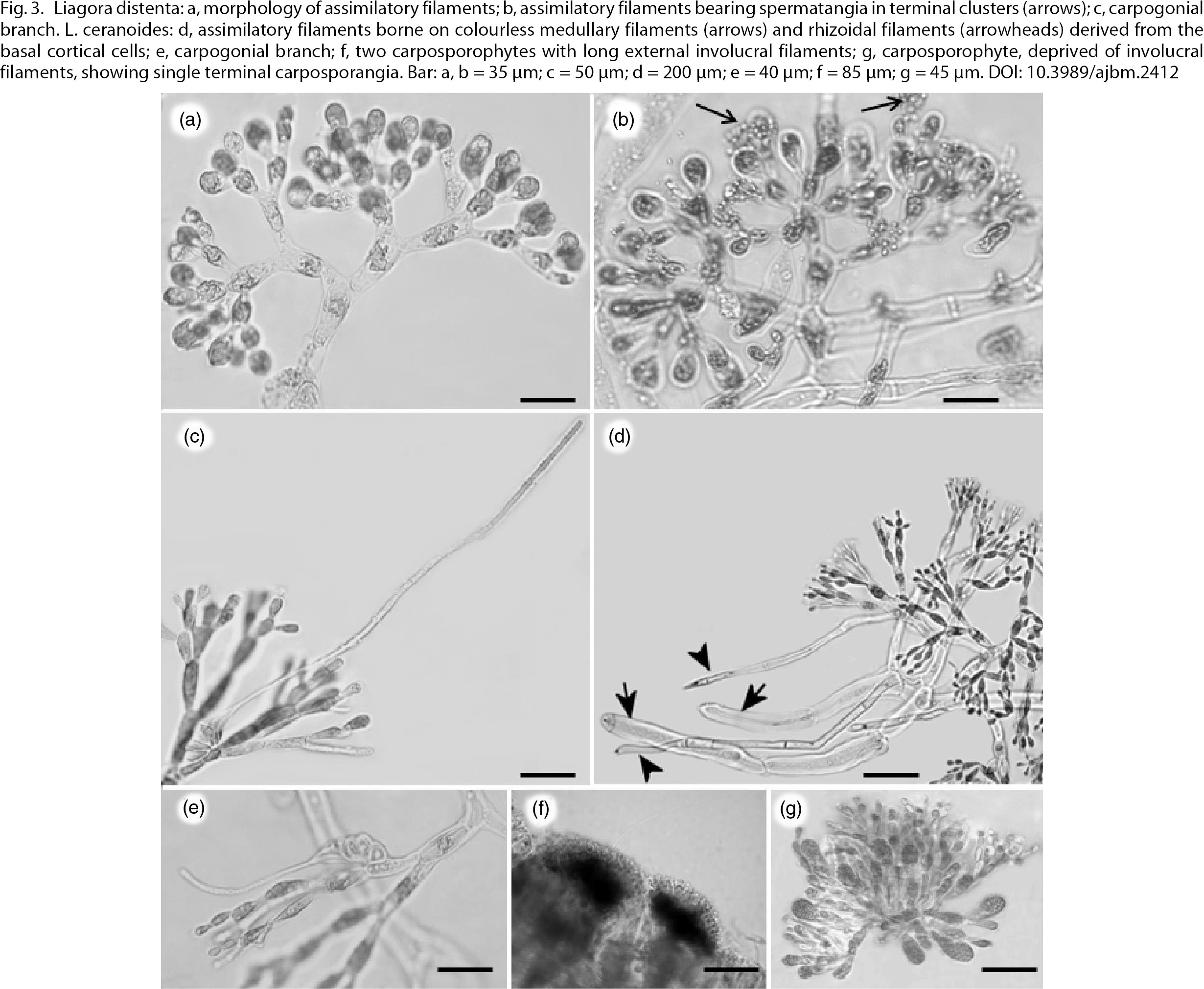 Fig. 3. Liagora distenta: a, morphology of assimilatory filaments; b, assimilatory filaments bearing spermatangia in terminal clusters (arrows); c,
carpogonial branch. L. ceranoides: d, assimilatory filaments borne on colourless medullary filaments (arrows) and rhizoidal filaments (arrowheads) derived from
the basal cortical cells; e, carpogonial branch; f, two carposporophytes with long external involucral filaments; g, carposporophyte,
deprived of involucral filaments, showing single terminal carposporangia. Bar: a, b = 35 μm; c = 50 μm; d = 200 μm; e = 40
μm; f = 85 μm; g = 45 μm. Fig. 3. Liagora distenta: a, morphology of assimilatory filaments; b, assimilatory filaments bearing spermatangia in terminal clusters (arrows); c,
carpogonial branch. L. ceranoides: d, assimilatory filaments borne on colourless medullary filaments (arrows) and rhizoidal filaments (arrowheads) derived from
the basal cortical cells; e, carpogonial branch; f, two carposporophytes with long external involucral filaments; g, carposporophyte,
deprived of involucral filaments, showing single terminal carposporangia. Bar: a, b = 35 μm; c = 50 μm; d = 200 μm; e = 40
μm; f = 85 μm; g = 45 μm.
|
|
Liagora ceranoides J.V. Lamour.
Moderately calcified fertile thalli, pink coloured with darker tips, up to 50 mm high, were collected in spring and summer.
The following features were useful for species identification: i) morphology of cortical assimilatory filaments composed of
oval cells becoming gradually shorter and slender (Fig. 3d); ii) abundant rhizoidal filaments produced from the basal cells of the assimilatory filaments (Fig. 3d); iii) structure and position of carpogonial branches (Fig. 3e), and iv) carposporophytes (Fig. 3f), showing compact gonimoblasts and long loosely arranged involucral filaments when mature (Fig. 3g). Our finding represents a new record to the Italian algal flora. In the Mediterranean Sea, L. ceranoides was previously recorded only from the Balearic Islands (Ribera-Siguán & Gómez Garreta, 1984) and Greece (Gerloff & Geissler, 1974), yet the Greek records require confirmation (Athanasiadis, 1987).
Pseudobryopsis myura (J. Agardh) Berthold ex Oltm.
Plants were collected at a depth of approximately 2 m in sheltered places. Thalli forming scattered feathery tufts bore typical
gametangia, separated from the supporting branches by a plug and with one apical papilla (Fig. 4a). This species was previously reported from Campania, Sicily, Veneto (Furnari & al., 2003) and Tuscany (Rindi & al., 2002).
|
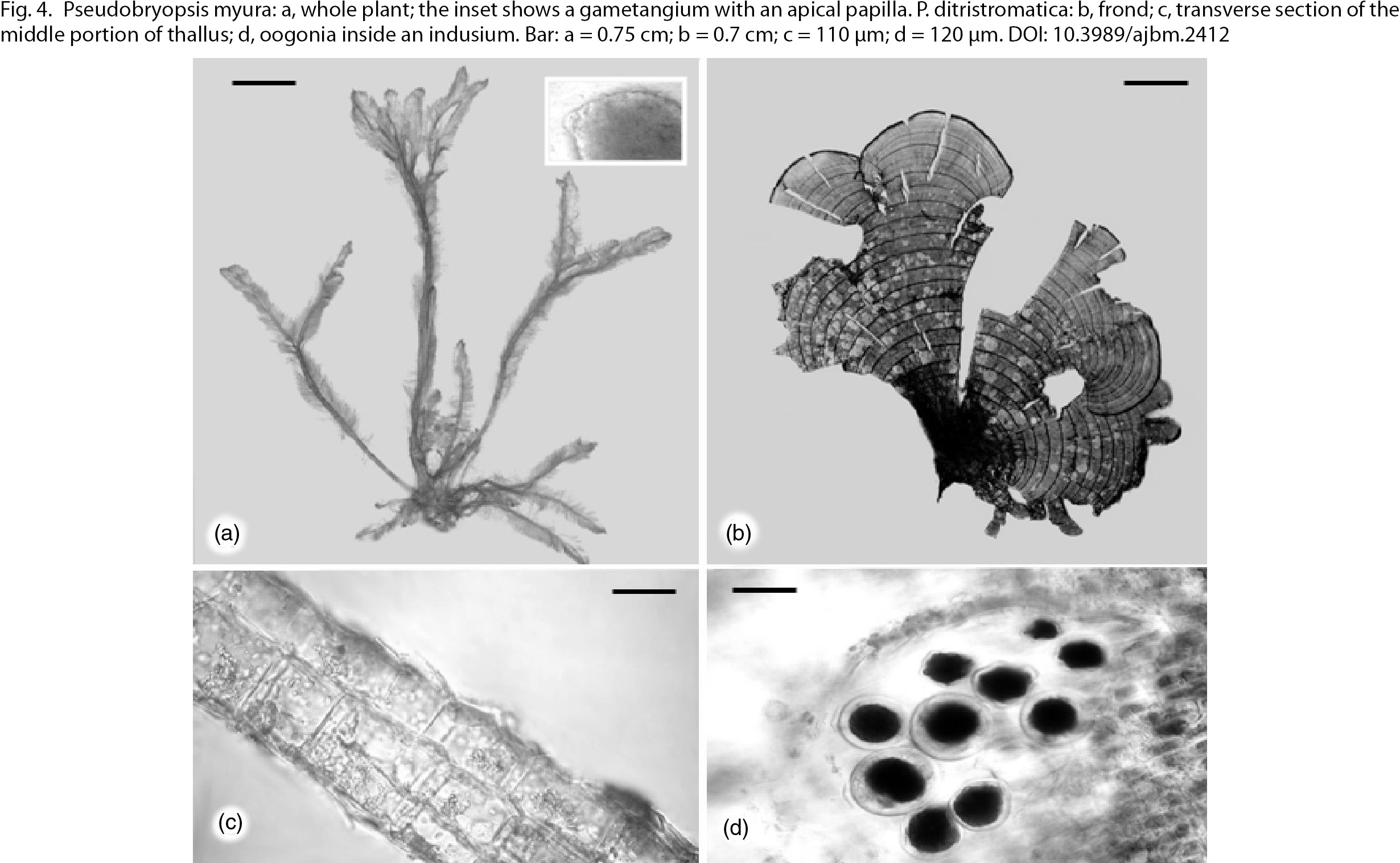 Fig. 4. Pseudobryopsis myura: a, whole plant; the inset shows a gametangium with an apical papilla. P. ditristromatica: b, frond; c, transverse section of the middle portion of thallus; d, oogonia inside an indusium. Bar: a = 0.75 cm; b = 0.7
cm; c = 110 μm; d = 120 μm. Fig. 4. Pseudobryopsis myura: a, whole plant; the inset shows a gametangium with an apical papilla. P. ditristromatica: b, frond; c, transverse section of the middle portion of thallus; d, oogonia inside an indusium. Bar: a = 0.75 cm; b = 0.7
cm; c = 110 μm; d = 120 μm.
|
|
Padina ditristromatica Ni-Ni-Win & H. Kawai
Female plants were collected at three sites from Santa Cesarea Terme; thalli were moderately to heavily calcified on both
surfaces; hair lines alternating between lower and upper surfaces were unequally spaced (Fig. 4b). Sections taken at the middle and basal portions showed a thallus composed of a mixture of 2–3 cell layers (Fig. 4c), a distinctive feature from P. pavonica (L.) Thivy. The oogonial sori were covered with an obvious indusium (Fig. 4d). Plants of this species were initially assigned to P. pavonica; accurate observations in the light of the recent paper on new Mediterranean Padina species (Ni-Ni-Win & al., 2011) confirmed that several of our specimens belong to the new taxon. According to Catra & Alongi (2013), it is very likely that P. ditristromatica may have a wider distribution in the Mediterranean Sea, because of possible misidentifications between the two species.
DISCUSSIONTOP
The present study documented approximately a 30% of the species previously recorded for the Apulian region. A comparison of
the number of species collected during the current survey with the corresponding numbers for other Apulian localities indicates
that the macroflora of Santa Cesarea Terme and Castro is relatively rich if the limited extent (approximately 9.5 km) of the
investigated area is considered. All the species recorded in the past from Santa Cesarea Terme (Huvé & al., 1963; Lazzo & al., 2002; Bottalico & al., 2011) were collected during this survey, with the only exception of Cystoseira barbata (Stackh.) C. Agardh and 25 records were new to this locality; 56 records from Castro were all new to that coastline. Six species,
previously reported from very restricted Apulian areas, represent the second record for the region, such as Acrosymphyton purpuriferum (J. Agardh) G. Sjöstedt that many years ago was collected only at the Tremiti Islands (Pignatti & al., 1967; Rizzi Longo, 1972), but it has no longer been found in the same locality (Cormaci & al., 2000). Twelve records are new to the Apulian Ionian coast, being previously reported only from the Adriatic side (Cormaci & al., 2001), 6 species are exclusive to the Ionian Sea and 149 species are shared by both seas. Only in one site of the investigated
coastline the infralittoral fringe community with Cystoseira amentacea var. stricta Mont. occurred; endemic to the Mediterranean Sea and typical of high hydrodynamic conditions it is recognised as an absolutely
protected species by Annex I of the Bern Convention (1979) and as a species of Community Interest in Annex V of Council Directive
92/43/EEC. Taking into account the loss of Cystoseira communities all over the Mediterranean basin (Pergent, 1991; Rodriguez-Prieto & Polo, 1996; Cormaci & Furnari, 1999; Thibaut & al., 2005; Mangialajo & al., 2008; Falace & al., 2010) and the possible decrease of the presence of this species, which is very sensitive to changes in water quality (Pinedo & al., 2007; Mangialajo & al., 2008), an analysis of spatial and temporal variations of our population would be needed. The occurrence along the investigated
coastline of two alien species (Acrothamnion preissii and Hypnea spinella) has not proved yet to impact negatively the indigenous flora; in fact, none of these species is characterised by invading
behaviour so far, even though continuous monitoring seems to be necessary.
The floras of the two caves of Santa Cesarea Terme, Grotta Grande and Grotta Solfatara, were different from that of Grotta
Palombara at Castro. This could be explained by the peculiar characteristics of the first two caves (i.e., very low light
conditions, the presence of suspended sulphureous material, which strongly reduces water transparency, and the unusual chemical
composition of the seawater), which favour the predominance of red algae, primarily Corallinales.
The R/P index (Rhodophyceae/Phaeophyceae) (Feldmann, 1937) of the surveyed coastal area (4.44) is higher than that determined for the Apulian (3.25) (Cormaci & al., 2001) and the Italian marine floras (2.50) (Furnari & al., 2010). On the other hand, it is very close to the values reported from other areas of the Ionian Sea: 4.39 for the Gulf of Taranto (Bottalico & Delle Foglie, 2003), 4.0 for Zakynthos Island (Tsirika & Haritonidis, 2005), and 4.1 for the Ionian versant of Apulia (Cormaci & al., 2001). Even higher R/P values were calculated for other Apulian localities, such as the Gargano promontory (5.1) (Cecere & al., 2000) and Porto Cesareo (6.29) (Cecere & al., 2005) and they were considered to reflect environmental perturbations. As comparable unstable conditions do not occur along the coastline from Santa Cesarea Terme to Castro, its high R/P ratio could actually represent evidence of an ongoing tropicalisation process. This concept was previously expressed by Lazzo & al. (2002) and reported by Falace & al. (2010), even if in the latter paper the coast of Santa Cesarea Terme was erroneously placed in the South Adriatic Sea. Even R/P values detected as low along the Greek coasts in the past (Tsekos & Haritonidis, 1977), because of the predominance of brown algae, have been found higher and approximately to 4.0 in more recent works (Tsirika & Haritonidis, 2005). The considerable occurrence of Indo-Pacific and Circumtropical elements in the chorological spectrum of the flora of the Ionian eastern coastline of the Peninsula Salentina, with percentage values much higher than those of the Apulian (Cormaci & al., 2001) and Italian (Furnari & al., 2010) floristic contingent, emphasises the affinities with floras of the eastern Ionian Sea. On the other hand, both the Ionian Sea and the southern Aegean Sea are considered as a wide refuge area for Circumtropical and Indo-Pacific species (Giaccone & Geraci, 1989).
Generally, the marine flora of the surveyed coastline has the following principal characteristics: i) extensive communities
of Corallinales, especially in the two sulphureous caves, that are ecologically significant, allowing colonisation by many
invertebrates and promoting an increasing biodiversity through the construction of coralligenous habitats; ii) limited presence
of species with a wide ecological valence; iii) occurrence of a restricted belt of the protected species Cystoseira amentacea var. stricta, supporting a rich associated flora, which would need to be carefully monitored over time.
Despite the presence of human-driven transformation, primarily associated with services and accommodation structures, this
coastline maintains a good ecological status and may represent an example of almost-intact ecosystem and an ideal reference
area for comparison with areas having higher levels of pollution.
ACKNOWLEDGEMENTSTOP
We thank Drs. G. Lazzo, C.I. Delle Foglie, G. Lapenna and A. Manghisi for their kind assistance with the field work and Prof.
G. Furnari for his critical reading of the manuscript. This work was supported by a grant from the University of Bari “A.
Moro”.
REFERENCESTOP
| ○ |
Alongi, G., Cormaci, M., Furnari, G. & Catra, M. 2012. Floristic macroalgal diversity in selected submarine caves located
within two marine protected areas off Lampedusa Island and Sicily (Italy). Botanica Marina 55: 387-397. http://dx.doi.org/10.1515/bot-2011-0072.
|
| ○ |
Andreakis, N., Procaccini, G. & Kooistra, W.H.C.F. 2004. Asparagopis taxiformis and Asparagopsis armata (Bonnemaisoniales, Rhodophyta): genetic and morphological identification of Mediterranean populations. European Journal of Phycology 39: 273-284. http://dx.doi.org/10.1080/0967026042000236436.
|
| ○ |
Ardissone, F. 1883. Phycologia mediterranea. Parte prima, Floridee. Memorie della Società Crittogamologica Italiana 1: 1-516.
|
| ○ |
Athanasiadis, A. 1987. A survey of the seaweeds of the Aegean Sea with taxonomic studies on species of the tribe Antithamnieae (Rhodophyta). University of Gothenburg, Gothenburg.
|
| ○ |
Boillot, A., Caram, B. & Meinesz, A. 1982. Sur l’Acrothamnion preissii Rhodophycée (Céramiales, Céramiacée) nouvelle pour la flore française. Cryptogamie, Algologie 3: 21-24.
|
| ○ |
Bottalico, A. & Delle Foglie, C.I. 2002. First record of Predaea ollivieri (Nemastomataceae, Rhodophyta) in Apulia (Southern Italy). Flora Mediterranea 12: 369-375.
|
| ○ |
Bottalico, A. & Delle Foglie, C.I. 2003. Contribution to the knowledge of the benthic marine flora along the eastern coastline
of the Gulf of Taranto (Ionian Sea). Flora Mediterranea 13: 261-272.
|
| ○ |
Bottalico, A., Delle Foglie, C.I. & Perrone, C. 2006. New records along the Apulian coasts. In: RAC-SPA (ed.), Proceedings of the Second Mediterranean Symposium on Marine Vegetation: 77-82. Tunis.
|
| ○ |
Bottalico, A., Lazzo, G. & Perrone, C. 2011. La flora marina di Santa Cesarea Terme (Lecce). Informatore Botanico Italiano 43(suppl. 1): 159.
|
| ○ |
Bottalico, A., Boo, G.H., Russo, C., Boo, S.M. & Perrone, C. 2014. Parviphycus albertanoae sp. nov. (Gelidiales, Rhodophyta) from the Mediterranean Sea. Phycologia 53: 243-251. http://dx.doi.org/10.2216/13-176.1.
|
| ○ |
Bottalico, A., Russo, C., Furnari, G. & Perrone, C. 2015. Parviphycus bompardii sp. nov. and P. albertanoae (Gelidiales, Rhodophyta), two species misidentified as Gelidiella ramellosa in the Mediterranean Sea. Phytotaxa 219: 155-164. http://dx.doi.org/10.11646/phytotaxa.219.2.5.
|
| ○ |
Catra, M. & Alongi, G. 2013. On some new and interesting marine macroalgae from the Greek coasts (Mediterranean Sea). Nova Hedwigia 97: 503-514. http://dx.doi.org/10.1127/0029-5035/2013/0124.
|
| ○ |
Cecere, E., Petrocelli, A., Saracino, O.D., Cormaci, M. & Furnari, G. 2000. Marine benthic flora of the Gargano promontory
(Adriatic Sea, southern Italy). Flora Mediterranea 10: 325-347.
|
| ○ |
Cecere, E., Petrocelli, A. & Saracino, O.D. 2005. Biodiversity of phytobenthic communities in the marine reserve of Porto
Cesareo. Biologia Marina Mediterranea 12: 78-87.
|
| ○ |
Cinelli, F. & Sartoni, G. 1969. Acrothamnion J. Ag (Rhodophyta, Ceramiaceae) genere algale nuovo per il mare Mediterraneo. Pubblicazioni della Stazione Zoologica di Napoli 37: 567-574.
|
| ○ |
Cormaci, M. & Furnari, G. 1999. Changes of the benthic algal flora of the Tremiti Islands (southern Adriatic) Italy. Hydrobiologia 398-399: 75-79. http://dx.doi.org/10.1023/A:1017052332207.
|
| ○ |
Cormaci, M., Furnari, G., Alongi, G., Catra, M. & Serio, D. 2000. The benthic algal flora on rocky substrata of the Tremiti
Islands (Adriatic Sea). Plant Biosystems 134: 133-152. http://dx.doi.org/10.1080/11263500012331358404.
|
| ○ |
Cormaci, M., Furnari, G., Alongi, G., Serio, D., Petrocelli, A. & Cecere, E. 2001. Censimento delle macroalghe marine bentoniche
delle coste pugliesi. Thalassia Salentina 25: 75-158.
|
| ○ |
Cormaci, M., Furnari, G. & Giaccone, G. 2004. Macrofitobenthos. In: Gambi, M.C. & Dappiano, M. (eds.), Mediterranean marine
benthos: a manual of methods for its sampling and study. Biologia Marina Mediterranea 11(Suppl. 1): 217-246.
|
| ○ |
Cormaci, M., Furnari, G., Catra, M., Alongi, G. & Giaccone, G. 2012. Flora marina bentonica del Mediterraneo: Phaeophyceae.
Bollettino dell’Accademia Gioenia di Scienze Naturali 45: 1-508.
|
| ○ |
Falace, A., Alongi, G., Cormaci, M., Furnari, G., Curiel, D., Cecere, E. & Petrocelli, A. 2010. Changes in the benthic algae
along the Adriatic Sea in the last three decades. Chemistry and Ecology 26: 77-90. http://dx.doi.org/10.1080/02757541003689837.
|
| ○ |
Feldmann, J. 1937. Recherches sur la végétation marine de la Méditerranée. La côte des Albères. Revue Algologique 10: 1-340.
|
| ○ |
Ferrer, E., Ribera, M.A. & Gómez Garreta, A. 1994. The spread of Acrothamnion preissii (Sonder) Wollaston (Rhodophyta, Ceramiaceae) in the Mediterranean Sea: new records from Balearic Islands. Flora Mediterranea 4: 163-166.
|
| ○ |
Furnari, G., Giaccone, G., Cormaci, M., Alongi, G. & Serio, D. 2003. Biodiversità marina delle coste italiane: catalogo del
macrofitobenthos. Biologia Marina Mediterranea 10: 1-482.
|
| ○ |
Furnari, G., Giaccone, G., Cormaci, M., Alongi, G., Catra, M., Nisi, A. & Serio, D. 2010. Macrofitobenthos. Biologia Marina Mediterranea 17: 801-828.
|
| ○ |
Gerloff, J. & Geissler, U. 1974. Eine revidierte Liste der Meeresalgen Griechenlands. Nova Hedwigia 22: 721-793.
|
| ○ |
Giaccone, G. & Geraci, R.M. 1989. Biogeografia delle alghe del Mediterraneo. Anales del Jardín Botánico de Madrid 46: 27-34.
|
| ○ |
Guiry, M.D. & Guiry, G.M. (eds.). 2014 [26th Nov]. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org.
|
| ○ |
Huvé, H., Huvé, P. & Picard, J. 1963. Aperçu préliminaire sur le benthos littoral de la côte rocheuse adriatique italienne.
Rapports et Procès-Verbaux des Réunions. Commission International pour l’Exploration Scientifique de la Mer 17: 93-102.
|
| ○ |
Klein, J.C. & Verlaque, M. 2011. Macroalgae newly recorded, rare or introduced to the French Mediterranean coast. Cryptogamie, Algologie 31: 111-130. http://dx.doi.org/10.7872/crya.v32.iss2.2011.111.
|
| ○ |
Kvaternik, D. & Afonso-Carrillo, J. 1995. The red algal genus Liagora (Liagoraceae, Rhodophyta) from the Canary Islands. Phycologia 34: 449-471. http://dx.doi.org/10.2216/i0031-8884-34-6-449.1.
|
| ○ |
Lazzo, G., Manghisi, A., Bottalico, A., Delle Foglie, C.I. & Perrone, C. 2002. Lista floristica preliminare delle macroalghe
di Santa Cesarea Terme (LE). Riassunti del 97° Congresso della Società Botanica Italiana, Lecce 24-27 settembre 2002: 36.
|
| ○ |
Lin, S.M., Huisman, J.M. & Payri, C. 2013. Characterization of Liagora ceranoides (Liagoraceae, Rhodophyta) on the basis of rbcL sequence analyses and carposporophyte development, including Yoshizakia indopacifica gen. et sp. nov. from the Indo-Pacific region. Phycologia 52: 161-170. http://dx.doi.org/10.2216/12-101.1.
|
| ○ |
Mangialajo, L., Chiantore, M. & Cattaneo-Vietti, R. 2008. Loss of fucoid algae along a gradient of urbanization, and structure
of benthic assemblages. Marine Ecology Progress Series 358: 63-74. http://dx.doi.org/10.3354/meps07400.
|
| ○ |
Ní Chualáin, F., Maggs, C.A., Saunders, G.W. & Guiry, M.D. 2004. The invasive genus Asparagopsis (Bonnemaisoniaceae, Rhodophyta): molecular systematics, morphology, and ecophysiology of Falkenbergia isolates. Journal of Phycology 40: 1112-1126. http://dx.doi.org/10.1111/j.1529-8817.2004.03135.x.
|
| ○ |
Ni-Ni-Win, Hanyuda, T., Draisma, S.G.A., Furnari, G., Meinesz, A. & Kawai, H. 2011. Padina ditristromatica sp. nov. and Padina pavonicoides sp. nov. (Dictyotales, Phaeophyceae), two new species from the Mediterranean Sea based on morphological and molecular markers.
European Journal of Phycology 46: 327-341. http://dx.doi.org/10.1080/09670262.2011.614355.
|
| ○ |
Occhipinti-Ambrogi, A., Marchini, A., Cantone, G., Castelli, A., Chimenz, C., Cormaci, M., Froglia, C., Furnari, G., Gambi,
M.C., Giaccone, G., Giangrande, A., Gravili, C., Mastrototaro, F., Mazziotti, C., Orsi-Relini, L. & Piraino, S. 2011. Alien
species along the Italian coasts: an overview. Biological Invasions 13: 215-237. http://dx.doi.org/10.1007/s10530-010-9803-y.
|
| ○ |
Pergent, G. 1991. Les indicateurs écologiques de la qualité du milieu marin en Méditerranée. Océanis 17: 341-350.
|
| ○ |
Perrone, C. & Delle Foglie, C.I. 2006. Parviphycus felicinii sp. nov. (Gelidiales, Rhodophyta) from South-East Italy. Cryptogamie, Algologie 27: 199-209.
|
| ○ |
Piazzi, L., Pardi, G. & Cinelli, F. 1996. Ecological aspects and reproductive phenology of Acrothamnion preissii (Sonder) Wollaston (Ceramiaceae, Rhodophyta) in the Tuscan Archipelago (Western Mediterranean). Cryptogamie, Algologie 17: 35-43.
|
| ○ |
Pignatti, S., De Cristini, P. & Rizzi, L. 1967. Le associazioni algali della Grotta delle Viole nell’Isola di S. Domino (Is.
Tremiti). Giornale Botanico Italiano 101: 117-126. http://dx.doi.org/10.1080/11263506709430741.
|
| ○ |
Pinedo, S., Garcia, M., Satta, M.P., De Torres, M. & Ballesteros, E. 2007. Rocky-shore communities as indicators of water
quality: a case study in the northwestern Mediterranean. Marine Pollution Bullettin 55: 126-135. http://dx.doi.org/10.1016/j.marpolbul.2006.08.044.
|
| ○ |
Ribera Siguán, M.A. & Gómez Garreta, A. 1984. Catálogo de la flora bentónica marina de las Islas Baleares, I (Rhodophyceae).
Collectanea Botanica 15: 377-406.
|
| ○ |
Rindi, F., Sartoni, G. & Cinelli, F. 2002. A floristic account of the benthic marine algae of Tuscany (Western Mediterranean
Sea). Nova Hedwigia 74: 201-250. http://dx.doi.org/10.1127/0029-5035/2002/0074-0201.
|
| ○ |
Rizzi Longo, L. 1972. La flora sottomarina delle Isole Tremiti. Atti dell’Istituto Veneto di Scienze Letteratura Arti, Classe di Scienze Matematiche e Naturali 130: 329-376.
|
| ○ |
Rodriguez-Prieto, C. & Polo, L. 1996. Effects of sewage pollution in structure and dynamics of the community of Cystoseira mediterranea (Fucales, Phaeophyceae). Scientia Marina 60: 253-263.
|
| ○ |
Silva, P.C. (ed.). 2014. Index nominum algarum, University Herbarium, University of California, Berkeley. http://ucjeps.berkeley.edu/INA.html
|
| ○ |
Thibaut, T., Pinedo, S., Torras, X. & Ballesteros, E. 2005. Long-term decline of the Fucales (Cystoseira spp. And Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Marine Pollution Bullettin 50: 1472-1489. http://dx.doi.org/10.1016/j.marpolbul.2005.06.014.
|
| ○ |
Thiers, B. (ed.) 2014. Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/
|
| ○ |
Tronholm, A., Steen, F., Tyberghein, L., Leliaert, F., Verbruggen, H., Ribera Siguán, M.A. & De Clerck, O. 2010. Species delimitation,
taxonomy and biogeography of Dictyota in Europe (Dictyotales, Phaeophyceae). Journal of Phycology 46: 1301-1321. http://dx.doi.org/10.1111/j.1529-8817.2010.00908.x.
|
| ○ |
Tsekos, I. & Haritonidis, S. 1977. A survey of the Marine Algae of the Ionian Islands, Greece. Botanica Marina 20: 47-65. http://dx.doi.org/10.1515/botm.1977.20.1.47.
|
| ○ |
Tsirika, A. & Haritonidis, S. 2005. A survey of the benthic flora in the National Marine Park of Zakynthos (Greece). Botanica Marina 48: 38-45. http://dx.doi.org/10.1515/BOT.2005.002.
|
| ○ |
Womersley, H.B.S. 1998. The marine benthic flora of southern Australia – Part IIIC. Ceramiales – Ceramiaceae, Dasyaceae. Australian Biological Resources Study & State Herbarium of South Australia, Canberra & Adelaide.
|
| ○ |
Zanolla, M., Carmona, R., De La Rosa, J., Salvador, N., Sherwood, A.R., Andreakis, N. & Altamirano, M. 2014. Morphological
differentiation of cryptic lineages within the invasive genus Asparagopsis (Bonnemaisoniales, Rhodophyta). Phycologia 53: 233-242. http://dx.doi.org/10.2216/13-247.1.
|
| ○ |
Žuljevic, A., Antolić, B. & Nikolić, V. 2009. Alohtone makroalge hrvatskog podmorja (Exotic marine macroalgae in Croatia).
10. Hrvatski biološki kongres s medunarodnim sudjelovanjem, Osijek, Zbornik sažetaka: 232.
|
Fig. 1. Map of Santa Cesarea Terme-Castro coastline showing sampling sites (CM: Contrada Malepasso; TS: Torre Specchialaguardia; Mi:
Miramare; Ar: Archi; PM: Porto Miggiano; PR: Porto Romanelli; Pa: Palombara) and the investigated marine caves (GG: Grotta
Grande; GS: Grotta Solfatara; GP: Grotta Palombara).